What is Cochrane?
Cochrane is an international organisation that summarises the best evidence from health research to help people make informed choices about treatments.
Cochrane’s mission is to provide accessible, credible information to support informed decision-making.
You can find out more about the organisation here.
What is Cochrane Crowd?
Watch our 2-minute animation about Cochrane Crowd to learn about what Cochrane Crowd is and how it works. Briefly, Cochrane Crowd is Cochrane’s citizen science platform. It hosts discrete tasks that identify and describe health research. Anyone is welcome to sign up and join this collective effort. You don’t need to be a health professional! Accompanying each task is a brief training module.
What tasks and activities are available on Cochrane Crowd?
When you first join up, you will be able to see our introductory tasks. As you progress, you will ‘unlock’ new tasks!
The main tasks we have are about helping us to identify certain types of health research called randomised trials. Don’t worry if you don’t know what a randomised trial is; each task is supported by a brief training module that will tell you what you need to know.
In addition to tasks, we have some stand-alone learning activities. We have some that are aimed at the complete beginner and we have other, more advanced modules.
How is the work I do in Cochrane Crowd of benefit to Cochrane?
The work that our contributors do in Cochrane Crowd is incredibly useful to Cochrane. With the tasks aimed at identifying reports of randomised trials, these records feed a unique and very important resource called the Cochrane Central Register of Controlled Trials (CENTRAL for short). This resource helps researchers find the randomised trials they are looking for quickly and easily. The more up-to-date CENTRAL is, the more accurate we can be in answering questions about the effectiveness of treatments.
To find out more about CENTRAL and how it is created click here.
To find out more about access to the Cochrane Library click here.
Where do the records in Cochrane Crowd come from?
Records come from a range of sources, some freely accessible such as those from ClinicalTrials.gov or PubMed. However some of our sources are from proprietary databases. At the moment we are working on records that have been sourced from Embase. Embase is owned by Elsevier, a for-profit publisher with whom our publisher, Wiley has a contract. This enables us to access and re-publish those records in CENTRAL. Wherever possible we seek to share the records identified by the community with as wide an audience as possible, within a framework that acknowledges and respects the legal rights and efforts of database providers.
What do I get out of this?
The Cochrane Crowd tasks are all designed to be doable for anyone with an interest in health. Some will take a bit longer to get the hang of it than others, but all are a great way to become more familiar with what is happening in the world of health research.
We’ve designed Cochrane Crowd so that as you cross certain milestones you are rewarded. For each task, you can progress through milestone badges!
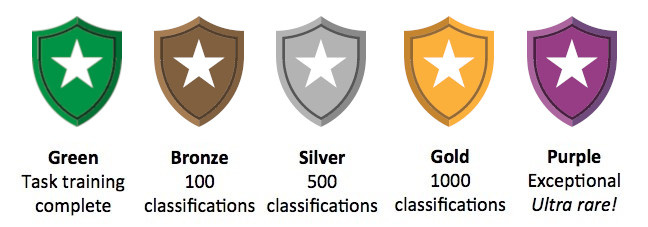
The badges will appear on your task dashboard as you achieve them. With each task you start off with the training module for that task. Once you complete it, you will earn your green badge. The next three badges, bronze, silver and gold, are awarded as you hit a certain number of classifications made. To achieve any of these badges is fantastic and you can aim to collect gold badges across all the Cochrane Crowd micro-tasks.
To earn your purple badge you’ll need to be truly exceptional at the task. It not expected that many people will reach this level. It requires a minimum of 1000 classifications combined with very high accuracy. Only around 1% of the Cochrane Crowd community has a purple badge.
In addition to earning your Cochrane Crowd badges, you can now also earn Cochrane membership through the work you do on Cochrane Crowd. The Cochrane Membership Scheme was launched in September 2017. This exciting initiative means that anyone can ‘join’ Cochrane, and your journey to greater contribution can start with Cochrane Crowd. 1000 classifications in Cochrane Crowd within a 12-month period will mean that you will be offered Cochrane membership.
In some tasks, you can earn named acknowledgement in a specific review. Depending on the task and the level of contribution, you may be asked to complete a brief conflict of interest form.
Finally, you will also be helping Cochrane in tasks that need doing. Cochrane is an international not-for-profit organisation. Our work is internationally recognised as the benchmark for high quality information about the effectiveness of health care. By helping us, you will become a part of that effort to produce high-quality, relevant, accessible systematic reviews and other synthesised research evidence.
What if I make a mistake?
You are bound to make a few mistakes, especially when you first start doing this. Even experienced screeners misclassify the odd record. We have created a very robust agreement algorithm to help counter this so don’t worry; no single record is screened by only one person and disagreeing decisions are arbitrated by expert screeners.
Can I use Cochrane Crowd for my students?
Yes, absolutely! We have built a tool called Classmate to help trainers, teachers and others use the Cochrane Crowd tasks for their students. You can access Classmate here. If you have any queries or questions about Classmate or about using Cochrane Crowd for your students, please do get in touch: classmate@cochrane.org.
Can I use Cochrane Crowd for my systematic review?
Cochrane review authors can access the Cochrane Crowd for their review under a new service called Screen4Me. If you think you would like to use Screen4Me for your Cochrane review, then get in touch with us at: s4m@cochrane.org.
Can I choose to work on records in particular topic areas?
Yes. You can do this from the Task preferences page. There you will see a tab called: My priority records list. Click on this tab and enter some terms or phrases that describe what you are interested in e.g. dementia, and we will prioritise records containing those terms for you. If you don’t get records containing any of those terms, it is because we don’t have any in the current batch being screened.
Can I promote Cochrane Crowd to people who are interested in certain healthcare conditions or diseases?
Yes. If you want to promote Cochrane Crowd to a group of people who will likely have a particular interest in a certain topic area, such as neonatal health, you can do that. We have created around 30 topic lists. To promote Cochrane Crowd at the topic level you just add the topic name to the end of the Crowd url. Staying with our neonatal example, if you want to let people know they can focus on records likely to be to do with neonatal health, you share this url: https://crowd.cochrane.org?filter=neonatal
Here are all the topics we’ve so far created:
Asthma (https://crowd.cochrane.org?filter=asthma)
Child health (https://crowd.cochrane.org?filter=childhealth)
Childhood cancer (https://crowd.cochrane.org?filter=childhood_cancer)
Colorectal cancer (https://crowd.cochrane.org?filter=colorectal_diseases)
Cystic fibrosis (https://crowd.cochrane.org?filter=cystic_fibrosis)
Dementia (https://crowd.cochrane.org?filter=dementia)
Diabetes (https://crowd.cochrane.org?filter=diabetes)
Ear, nose and throat (https://crowd.cochrane.org?filter=ent)
Epilepsy (https://crowd.cochrane.org?filter=epilepsy)
Eyes and vision (https://crowd.cochrane.org?filter=eyes)
Fertility (https://crowd.cochrane.org?filter=fertility)
Heart (https://crowd.cochrane.org?filter=heart)
HIV/AIDS (https://crowd.cochrane.org?filter=HIV)
Hypertension (https://crowd.cochrane.org?filter=hypertension)
Incontinence (https://crowd.cochrane.org?filter=incontinence)
Infectious diseases (https://crowd.cochrane.org?filter=infectious_diseases)
Injuries (https://crowd.cochrane.org?filter=injuries)
Learning problems (https://crowd.cochrane.org?filter=learning_problems)
Lung cancer (https://crowd.cochrane.org?filter=lung_cancer)
Movement disorders (https://crowd.cochrane.org?filter=movement)
Multiple sclerosis (https://crowd.cochrane.org?filter=ms)
Neonatal (https://crowd.cochrane.org?filter=neonatal)
Neuromuscular (https://crowd.cochrane.org?filter=neuromuscular)
Oral health (https://crowd.cochrane.org?filter=oral_health)
Palliative care (https://crowd.cochrane.org?filter=palliative)
Pregnancy (https://crowd.cochrane.org?filter=pregnancy)
Schizophrenia (https://crowd.cochrane.org?filter=schizophrenia)
Skin (https://crowd.cochrane.org?filter=skin)
Stroke (https://crowd.cochrane.org?filter=stroke)
Tobacco addiction (https://crowd.cochrane.org?filter=tobacco_addiction)
Wounds (https://crowd.cochrane.org?filter=wounds)
If you would like us to create one for you, just get in touch: crowd@cochrane.org
What if I sign up and then realise I don’t really want to do it after all?
This will rip a hole in the space-time continuum and it’ll be hard to put right. I’m only joking. If you sign up and then find you don’t want to do this after all, or you simply don’t think you have the time, then that is absolutely fine. You don’t need to feel bad or to notify us. We expect that some will sign up and then realise that actually it’s not for them after all.
Can I screen on my tablet or my smart phone?
Cochrane Crowd is web-based but many of the tasks work well on tablets and on phones.
Can I work offline?
Yes. The way Cochrane Crowd works is that a small number of records are downloaded for you automatically. These are then automatically synced with the server. If you know you’ll be offline for a while and want to do this task, then you can select to download up to a 1000 records to work on. This feature is accessible from your Settings.
Can I feedback my thoughts to the Cochrane Crowd team?
We would love to hear your feedback on any aspects of Cochrane Crowd. Please don’t hesitate to contact us at crowd@cochrane.org.